Electroplating Experimentzzzz
Ingrediants:
Conductive coating:
Electroforming non conductive objects :
-
Conductive Paint (Alcohol based paint )
-
Gold leaf
-
Conductive adhesive fabric
-
Isopropyl Alcohol (90%+) for cleaning organic elements
-
Metal Cleaner
Electrolytic solution :
- copper sulphate 100g (for crystals) --> better use 200g for more electroplating
- Hot water 500 ml (or 1000 ml for 200g)
Homemade electrolytic solution:
- hydrogene peroxide 50% 50% 250 ml
- Vinegar 14%
--> This wil etch the copper of your copper but takes much longer (1-2 days) before electroplating
Tools:
- végetable glycerine
- glue
- gloves
- 1.5 v 0.06A power supply
- paper towls
- crocodile clips
- carbone rod (for cathode - )
- Copper plate or thick copper wire (for anode +)
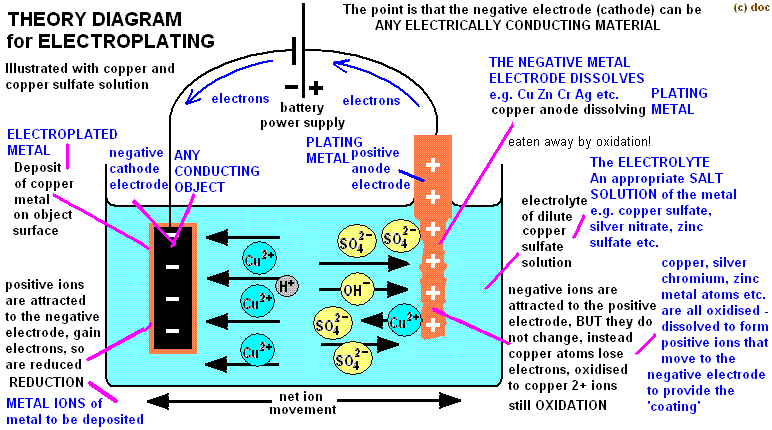
You can electroplate anything conductive, if you want to electroplate something none conductive (actually called electroforming) you need to apply a conductive coating on it.
Conductive coating tested:
- I tested a graphite spray (quite resistive) but it fried and dissolved after some time so maybe need to apply a glue before and then spray graphite.
- Bare conductive paint electroplated a little but not very satisfying...the carbone rod was more conductive so it electroplated the rod mainly..
- Gold leaf works well
- Silver conductive thread works well


Preparing the solution
- Prepare the electrolytic solution by mixing 200g copper sulphate +1000ml warm water to dissolve
- Add with crocodile clips the anode + in the solution (not entirely sinked) attaching to it a pièce of copper (thick copper wire, or rod or plate..)
- Add with crocodile clips the cathode - in the solution (entirely sinked) and attach the object you want to electroplate. Test continuity with multimeter to be sure their is a strong connection between the 2 elements.
- Attach anode to positive terminal of 2 AAA battery (1.5+1.5 v = 3 V)
- Attach cathode to negative terminal of the battery
- Leave for at least 1 hour and check regularly
- Rince with water




Other tests:
Homemade solution of 50% vinegar + 50% hydrogene peroxyde. Really good for etching out conductive fabric too..
The greenish powder can also be used to create semiconductors (homebrew cuprous oxyde diode H. P. Friedrichs ) used for creating diodes for crystal radios in the 1900's..




Sources:
http://www.docbrown.info/page01/ExIndChem/electrochemistry04.htm
http://alain.vassel.pagesperso-orange.fr/electrochimie.htm
https://www.youtube.com/watch?v=Nx-GwKOH5qc&feature=youtu.be
Next step: <3 <3


Source: http://www.wholesalegoldvials.com/2012/10/02/silver-and-copper-crystals/
"How do you grow such beautiful copper and silver crystals?
First we start with an electrolytic refining cell. This kind of cell is generally used to refine silver, copper, and gold. But during the refining process silver and copper are capable of forming crystals. Gold will not crystallize it only plates out, which works great for refining but isn’t nearly as beautiful.
Next we create our solution. When growing silver crystals we take and dissolve pure silver granules into nitric acid and purified water using a boiling process. The process is complete when the granules are dissolved and the PH has risen from a 0 to a PH of 4. This solution is called an electrolyte solution and contains approximately 20 oz of silver per gallon.
Now we take our electrolyte solution and pour it into the cell. A cell should be anywhere from two to four gallons. Then we take and submerge our plates. One plate is at least 92% pure silver and the other is stainless steel. Once the plates are submerged and secured we hook up the electricity. We attach the positive lead to the silver plate (anode) and the negative lead to the steel plate (cathode). Then using a DC trickle charge we run approximately one and a half volts through the cell.
As the electricity runs through the cell the plate of pure silver starts to slowly dissolve. But as fast as the silver is dissolving into the solution the silver is being deposited onto the steel plate in the form of crystals. This is called an ion exchange.
As the silver plate is dissolving it gives off a black sludge so a fine cloth bag is placed around the plate to collect the sludge for easier cleaning. This sludge contains all of the impurities from the silver plate. In this process an impurity is anything that isn’t silver such as gold, tin, zinc, and lead, but not copper. Copper is dissolved into the solution. So when the solution turns a dark blue color it is time to discard it, but not before the silver is removed to be used again.
It takes about 24 hours for average crystals to form and three to four days for the bigger heavier ones. Once the crystals reach the desired size the plates are removed from the solution and the crystals are scraped off and cleaned with distilled water and allowed to air dry.
There are several factors that influence how a crystal will form. The amount of electricity running through the cell, the amount of silver in the solution, even the temperature (the crystals prefer the warmer temperatures).
The process for creating copper crystals is the same as with the silver, we simply switch out the silver and steel plates for copper ones and the silver granules with copper wire. And to create the copper crystals on the pennies we simply attach some copper pennies to one of the plates and watch them grow. "